Enzymes are special biomolecules that catalyse biochemical reactions. Almost all enzymes are proteins i.e. chains of amino acids linked together. Enzymes catalyse chemical reactions by lowering the activation energy of chemical reactions inside the cell. The free energy of reactant or product however remains unaffected by enzymes. Enzymes show high specificity i.e. A particular enzyme can only act on one substrate.
1.1. History of Enzyme study:
E. Buchner successfully extracted an enzyme from the juice of yeast cells which catalyzed alcoholic fermentations, in 1897. He coined the term ‘enzyme’. Soon other extracts of similar nature were discovered and also termed as enzymes. In 1926, J. B. Summer isolated an enzyme in a pure crystalline form for the very first time. The enzyme he isolated was Crease, from the extract of Jack-bean. Between the years 1930 and 1936, the enzymes pepsin, trypsin, and chymotrypsin were crystallized by J. Northrop to prove their protein nature. At present time, more than 3000 enzymes are known and at least 250 of them have been purified. 1
1.2. Energy of Activation (EA):
The energy of activation is defined as the amount of energy, in calories, required to bring all the molecules of one mole of the substrate at a given temperature to its activated, transient state. A transition state is the energy-rich state of the interacting molecules at the top of the energy barrier. The rate of reaction depends upon the number of molecules having activation energy to react together at any moment.
1.3. Chemical nature and properties of enzyme:
Almost all enzymes are invariably protein. However, recently, scientists have discovered that a few RNA molecules also function as enzymes. The structure and conformation of an enzyme is essential for its catalytic activity. The functional unit of the enzyme is called holoenzyme and is made up of apoenzyme (the protein part) and co-enzyme (the non-protein organic part). If the enzyme is made up of a single polypeptide, the enzyme is known as a monomeric enzyme. Eg. Ribonuclease, Trypsin. Enzymes which possess more than one subunit are called oligomeric enzymes. Eg. Lactate dehydrogenase, Aspartate transcarbamoylase, etc.2
General Properties of an enzyme:
- Enzymes are responsible for accelerating the rate of reaction by lowering the energy of activation.
- Enzymes contain a particular pH at which their efficiency is maximum called optimum pH.
- The substrate reacts with a highly precise area (the active site) to produce the desired product.
- Enzymes remain intact throughout the process of a chemical reaction.
- Enzymes contain a particular temperature at which their efficiency is maximum called optimum temperature.
- All enzymes are proteins but all proteins may not be enzymes.
1.4. Mechanism of Action of Enzymes:
As already mentioned above, an enzyme catalyzes biochemical reactions by lowering the energy barrier of reactants. Enzymes do not alter the equilibrium constant of a reaction; they only enhance the velocity of the reaction. Lowering activation energy makes the given reaction possible at a lower temperature. It is due to enzymes that all the biological systems occur at body temperature (below 40 ºC).
Formation of Enzyme – substrate complex:
The fundamental principle of enzyme catalysis is that the substrate(S) has to combine with the enzyme(E) at the active site to form an enzyme-substrate complex, which ultimately leads to product formation(P).
E + S

E – S complex

P (Product)
Various theories have been put forward to explain the mechanism of enzyme-substrate complex formation. Among them, the most accepted theories regarding enzymatic action are as follows:
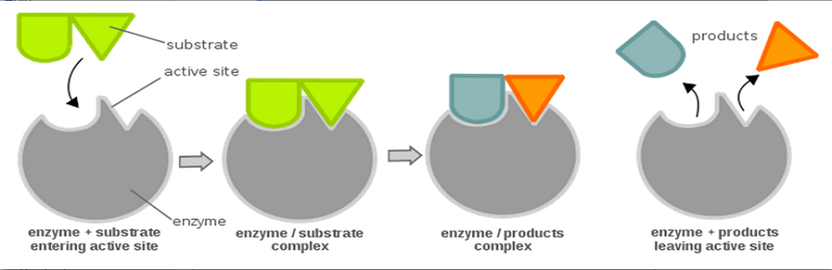
Lock and Key model or Fischer’s template theory:
This theory was proposed by German biochemist Emil Fischer. According to his theory, enzymes are rigid in nature, and the substrate fits into the binding site just as a key fits into the proper lock. The active site of an enzyme is a rigid and pre-shaped template where only a specific substrate can bind. This model has drawn heavy criticism because it fails to explain many aspects of enzymatic reaction, chiefly the effect of allosteric modulators.
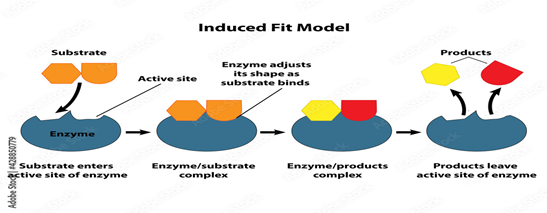
Induced Fit theory or Koshland’s theory:
This model was designed by Koshland in 1958. According to him, the conformational change of the active site is not rigid and pre-fixed. The interaction of the substrate with the enzyme causes a conformational change in the enzyme, resulting in the formation of the required substrate binding site. The appropriate amino acids of the enzymes are repositioned to form the active site and induce catalysis. This model has been experimentally proven through X-ray diffraction studies.
Substrate strain theory:
According to this model, the substrate is strained due to the induced conformational change in the preformed active site of the enzyme. The strained substrate leads to the formation of the product.2
1.5. Factors affecting the rate of enzyme activities:
The rate of enzyme-catalyzed reactions depends upon various factors some of which are; pH, temperature, enzyme concentration, substrate concentration, and product concentration.
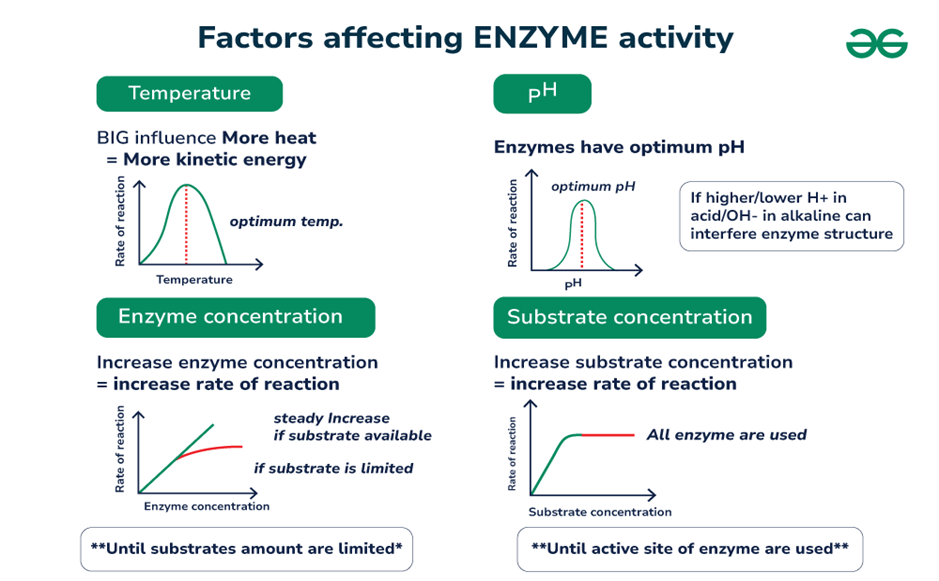
- pH: Enzymes are proteins and contain amino groups as well as carboxyl groups. pH change affects the ionic character of the amino and carboxyl groups. Most enzymes function most effectively at an optimum pH (usually 7 +/- 1.5). Enzymatic activity declines as the pH level rises or drops from the optimum pH. However, Pepsin has unusually low optimum pH due to its adaptation to acidic conditions of the stomach where it functions.
- Temperature: The rate of reaction generally increases within a certain range on increasing the temperature until the enzyme is stable and retains its full activity. The rate of enzyme-catalyzed reactions approximately doubles for every 10 ºC rise in temperature. Temperature coefficient (Q10) varies from one enzyme to another depending upon the energy of activation of Enzymes. Most enzymes rapidly denature above 55-60 ºC due to the loss of tertiary and quaternary conformations. However, some thermophilic bacteria can survive in hot springs above 85 ºC and contain functional enzymes.
- Effect of enzyme concentration: The rate of enzyme-catalyzed reaction is directly proportional to enzyme concentration i.e. rate of reaction increases on increasing the enzyme concentration.
- Effect of product concentration: In many multi-enzyme systems, the end product can inhibit the first enzyme to maintain constant self-regulation. Thus, the rate of the entire sequence is determined by the steady-state concentration of the product.
- Effect of substrate concentration: On increasing the substrate concentration, the rate of enzyme-catalyzed reaction increases until all the available active sites are occupied by the substrates. Once all the active sites are used up, the rate of reaction remains constant with a further increase in substrate concentration.
1.6. Classification of enzymes:
The number of enzymes present in the biological system is massive. To make its study easier, the Enzyme Commission of the International Union of Biochemistry (1924) has classified enzymes on the basis of the type of reaction catalyzed by them. According to this system, enzymes are classified into six major classes; Oxidoreductases, Transferases, Hydrolases, Lyases, Isomerases, and Ligases.
| Enzyme | Type of reaction catalysed | Example |
| Oxidoreductases | Biological reduction and oxidation. | Dehydrogenases, Oxidases, Peroxidases |
| Transferases | Transfer of a biologically important group from one molecule to another. | Transaminases, Kinases, Phosphorylases |
| Hydrolases | Splitting of molecules into two by action of water. | Peptidases, Phosphatases, Glycosidases |
| Lyases | Type of reaction catalyzed | Carboxylases, Aldolases, Decarboxylases |
| Isomerases | Conversion of one isomer to a different form by redistribution of atoms. | Mutases, Racemases, Epimerases |
| Ligases | Linking together of molecules at the expense of ATP or a similar triphosphate. | Synthetases |
1.7. Regulation of enzymes:
Enzymes carry out thousands of biological activities within the cell, which must be carefully controlled. They can be regulated in such a way that can either promote or reduce their activity. There are various molecules that promote or inhibit enzyme activity and various mechanisms to do so.
Those compounds that have the ability to combine with the enzyme reversibly or irreversibly to block the catalysis by that enzyme are known as inhibitors. Some inhibitors are similar to substrate molecules. They bind to the active site and block the substrate from binding. This phenomenon is known as competitive inhibition. In non-competitive inhibition, the inhibitor molecules bind to the enzyme at an allosteric site, a binding site away from the active binding site. Some inhibitors bind to an enzyme in a location where it induces a conformational change that reduces the affinity of the enzyme toward the incoming substrate. This type of inhibition is called allosteric inhibition.3
Many enzymes cannot work at the optimal level until they bind with other specific non-helper molecules i.e. cofactors and coenzymes. Cofactors are non-protein component that actively promotes enzymatic reactions. They do not have catalytic ability on their own. They may undergo certain temporary conformational changes during reaction but they are normally restored afterwards. Inorganic ions such as Iron, Magnesium, Manganese, Cobalt, Zinc, etc act as cofactors.
Coenzymes are small heat-stable organic molecules, which bind reversibly and transiently to an enzyme. The most common sources of coenzymes are known to be dietary vitamins. Some of the coenzymes derived from vitamins are as follows;
| Coenzyme | Vitamin |
| Thiamine pyrophosphate | Thiamine |
| Flavin mononucleotide | Riboflavin |
| Coenzyme A (CoA) | Pantothenic acid |
| Nicotinamide Adenine Dinucleotide(NAD) | Nicotinic acid |
| Coenzyme B12 | Vitamin B12 |
REFERENCES
- S.C. Rastogi. Cell and Molecular Biology. Third edition. New Age International Publishers
- Satyanarayan U, Chakrapani U. Biochemistry. Fourth. Reed Elsevier Private Ltd & Books and Allied (P) Ltd.; 2013.
- Clark MA, Choi J, Douglas M. Biology 2e. Second. The Rice University Press; 2020.