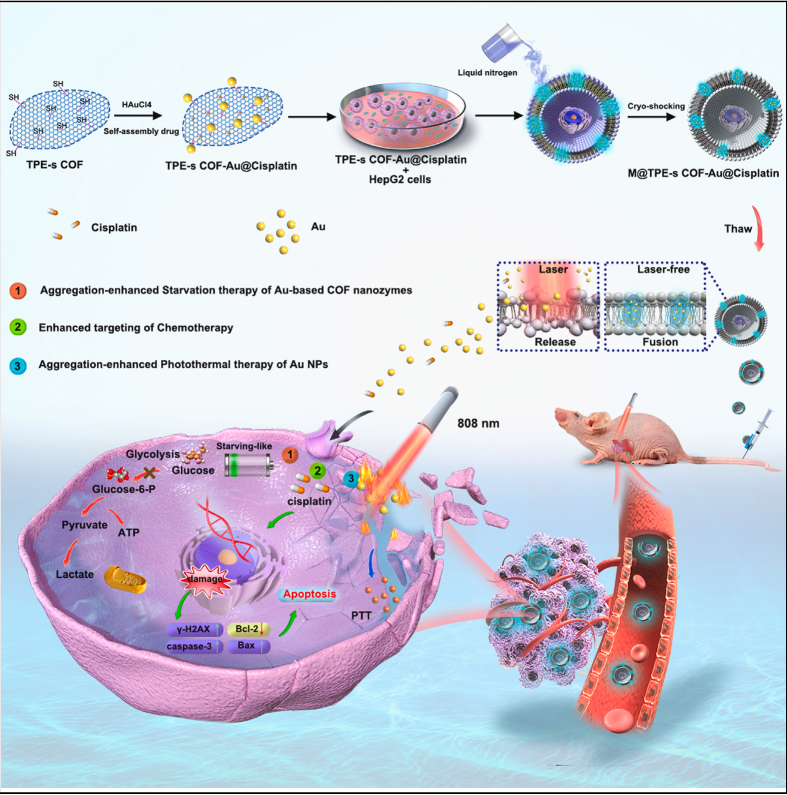
A Smarter Way to Fight Cancer
Cancer therapies often face a double challenge: destroying tumor cells while sparing healthy tissue. Traditional chemotherapy is powerful but nonspecific, while newer treatments like photothermal therapy can lack precision. To bridge this gap, scientists have designed an innovative solution—tumor-derived covalent organic framework (COF) nanozymes that deliver a one-two punch of chemo- and photothermal therapy.
Recycling Tumors into Weapons
What makes this approach remarkable is the source of the material: the nanozymes are built using components derived directly from tumors. These tumor-origin elements ensure the nanozymes inherit natural tumor-homing capabilities, allowing them to accumulate more effectively in cancerous tissues compared to synthetic nanoparticles.
At their core, the COF nanozymes act like artificial enzymes, catalyzing reactions that generate reactive oxygen species (ROS). When exposed to near-infrared light, they also trigger localized heating, further stressing and killing tumor cells.
Combination Therapy in Action
The nanozymes were loaded with the chemotherapy drug doxorubicin (DOX), creating a powerful chemo-photothermal platform. In preclinical tests, this system showed:
- Precise tumor targeting thanks to inherent tumor-derived signatures.
- Dual-action killing via drug release and photothermal heating.
- Enhanced ROS production, overwhelming cancer cells’ defenses.
- Reduced side effects, since healthy tissues were largely spared.
This strategy not only maximized tumor-killing efficiency but also minimized systemic toxicity—a long-standing challenge in oncology.
Why It Matters
- For patients: Potential for safer, more effective treatment with fewer side effects.
- For oncology: A new paradigm that merges biomimicry with nanotechnology.
- For science: Proof-of-concept that tumors can be repurposed against themselves.
By uniting materials science, enzymology, and oncology, tumor-derived COF nanozymes highlight how interdisciplinary innovation can open new frontiers in precision medicine.
Conclusion
This research marks a step toward a future where cancer therapies are not only more targeted but also more intelligent—leveraging the tumor’s own biology against it. With further development, tumor-derived nanozymes could redefine combination therapy and bring us closer to safer, personalized cancer care.
Reference
Zhou, S., Tian, T., Meng, T., Wu, J., Hu, D., Liao, Q., … & Zhang, G. (2023). Tumor-derived covalent organic framework nanozymes for targeted chemo-photothermal combination therapy. IScience, 26(8). 10.1016/j.isci.2023.107348